Genetic Testing, at Your Nearest Clinic Today!

In the field of biology, the last century began with the inception of genetics as a subject of scientific inquiry, and it ended with the ambitious Human Genome Project. Our genome is the sum of our entire genetic endowment, containing all the instructions needed to build and maintain us. It is present in every cell in our body in the form of DNA. In an astonishing way, most of our resultant complexities are coded from combinations of only four different chemical constituents or bases of DNA. These four bases are individually repeated 3 billion times in a unique sequence, forming what we know as our DNA and our genome. The goal of the Human Genome Project was to determine this entire 3 billion base sequence. That endeavour, often compared in its ambition to the moon landing, aspired to simplify the mysteries of human life into a sequence of code assembled by a mere four letters.
Ever since the completion of the project in 2003, there has been speculation about how insights from genomics will be used to advance healthcare and medicine. New slogans to reflect and anticipate these changes keep appearing in mass media, such as ‘the future of medicine is in genomics’ and ‘the era of genomics is here’. 1 From growing spare body parts just for you, to creating personalised diets, it seems breakthroughs and innovations are looming just around the corner, waiting to be put to use. And rightly so, this ever-developing field is brimming with potential. Given the rapid pace of advancement, it is difficult, even for a close observer, to discern the present reach of the field from its future capabilities. It is estimated to take 17 years for a novel scientific finding to reach routine clinical use, 2 so it should not be surprising to learn that most genomics-related innovations haven’t yet reached the clinic. However, these innovations are currently fueling developments in some of the already established clinical areas, such as in the case of genetic testing.
Today, many hospitals around the world have a dedicated genetics department, even though its current use is restricted mainly towards genetic testing and counselling. Here, trained professionals discuss with their patients—many of whom are prospective parents—the genetic aspects of concerned illness and advise them regarding genetic testing and its interpretation. Most genetic tests involve limited physical risk as only a blood sample or cheek swab is needed. However, there are currently several limitations of genetic testing in terms of its clinical utility and reliability of results. As more and more clinical data is collected, coupled with developments in genetic research, these aspects are set to improve over time as the use of these tests becomes more routine.
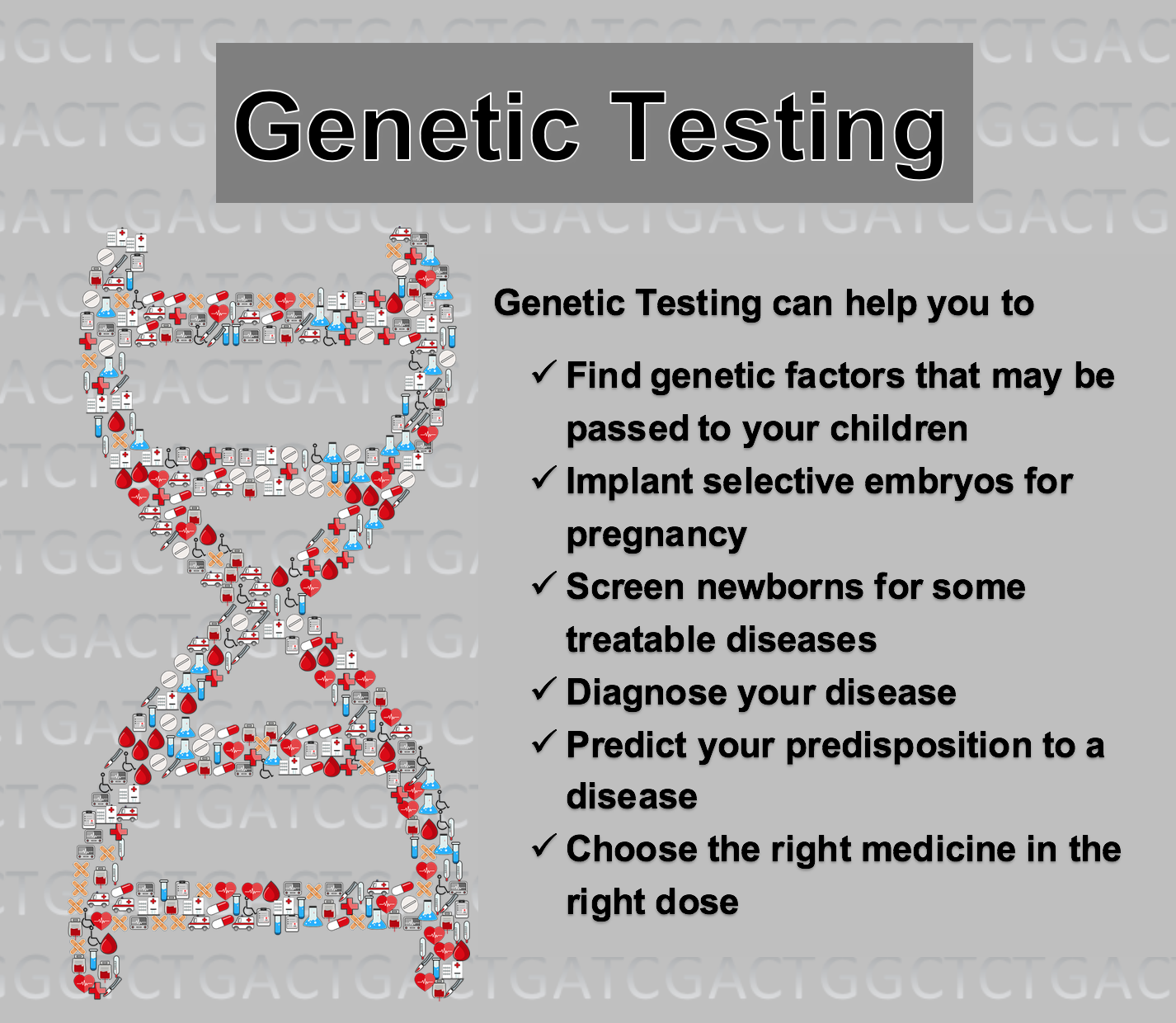
Within genetic testing there are currently a wide range of applications available, spanning the entirety of human life. Even before a child is conceived, couples with a family history of recessive genetic disorders are recommended to undertake carrier testing. This identifies individuals who may not have any physical symptoms but might still be carrying one of two copies of the disease-causing gene. If one of the partners is found to be a carrier, he or she has a 50% chance of passing that aberrant gene to each of its offspring. In this way, carrier testing is limited— it can only determine the risk of passing on the disease-causing gene, and does not predict if the child will receive the gene or not. In several countries, women who are planning a pregnancy are offered carrier screening for certain genetic disorders with the option of additional screening for targeted conditions or expanded carrier screening as well. In the UK, you need a referral from your GP or specialist to carry out such genetic testing. In these cases, in addition to family history, another rationale to opt for genetic testing is if you belong to an ethnic group that has been found to have a higher predisposition to certain genetic disorders.
If one or both partners are suspected to be carriers or have family histories with conditions of known genetic basis, they are advised to opt for assisted reproductive technology, such as in vitro fertilisation (IVF), instead of natural pregnancy. Here, preimplantation genetic diagnosis (PGD) can be applied to first screen the genome profile of the embryos. Only the embryos without the suspected genetic condition are then implanted into the mother for pregnancy. Such selective implantation reduces the risk of conceiving a child with the suspected genetic condition. With its increased use in recent years, this type of testing has raised several ethical issues. When are parents justified in discarding the embryos? Some groups are concerned that increased genetic testing will cause the selective extinction of conditions from the population. What about those genes that only increase the risk and cannot predict if a disease condition will manifest? Many believe that people have a moral duty to spare the next generation, their children, from a life of suffering. Also, the use of this technology is not limited to bringing about changes that impact our health directly, and can be used, for example, to choose the sex of the child we wish to have. A hefty price tag is also currently a barrier in many parts of the world. In the UK, PGD is highly regulated and a committee decides if a condition is serious enough to allow intervention on a case by case basis. PGD is currently approved for more than 600 conditions in the UK and is available through the NHS if certain conditions are met. 3
Once the foetus is conceived, prenatal genetic testing is available to screen for the suspected genetic condition in the developing baby before it is born. Here, it is possible to determine with greater accuracy if the foetus is carrying the disease-causing gene or not. This helps to better inform decisions for intervention and management of care. However, there is a small health risk involved with prenatal testing that may even lead to miscarriage. As of last year, 4,451 genes out of our complete repertoire of approximately 22,000 genes had been identified to carry a disease mutation. Preimplantation and prenatal diagnosis are now available for almost all of these monogenic disorders, these are disease conditions caused by change in a single gene 4. As advancements further our understanding of the underlying genetic basis of many more diseases, rendering them available for genetic testing, it is important to keep in mind both the benefits and the risks.
After birth, newborn genetic screening looks for certain genetic disorders that can benefit from diagnosis and treatment early in life, such as phenylketonuria and congenital hypothyroidism. In the US, newborn screening covers a wide-range of conditions that have been selected based on multiple criteria including burden of the disease, cost versus benefits, and availability of treatment. As a result, every baby born today is tested at birth for 34 to 60 inherited or congenital genetic diseases 5. While in the UK, with its own disease statistics, the focus has been narrower, and genetic screening at birth is limited to nine rare but serious genetic conditions. 6
This comparatively lower number in the UK is due to a lack of confidence in the net benefit of screening, perhaps because of apprehension surrounding its negative impact on overdiagnosis, including false positives and indeterminate results.
7Moving on to the subsequent developmental years of the child, there are several disease conditions for which a clear clinical diagnosis is not available. This is the case in the many types of cognitive developmental disorders, including autism spectrum disorder. With genetic diagnostic testing, it is possible to identify or rule-out such conditions more accurately. Whole exome tests are now being carried out at an increasing rate whenever doctors are uncertain about diagnosis. Here, instead of looking at specific genes, this single test analyses all the genes of an individual and highlights the genetic changes that could be causing the disorder.
For a number of disease conditions, treatments that are currently available show a disparity in how they affect individual patients. Many of these differences in response are the result of individual genomic variations. In such cases, pharmacogenomic testing helps to determine if a particular therapy or dose of therapy will be effective for an individual patient by considering their genomic information. Currently, there are more than 100 FDA-approved drugs containing pharmacogenomics-related information on their labels and for which genetic testing is recommended to determine suitability. These drugs cover diverse fields of applications such as antivirals, cardiovascular and anti-cancer therapeutics.8
Finally, many genetic diseases appear later in life and, in such cases, predictive or pre-symptomatic testing can be used to determine the risk of developing these before any signs or symptoms appear, such as with certain types of cancer, Alzheimer’s, and Huntington’s disease.
Caption: The many uses of genetic testing today
Information about more than 50,000 genetic tests covering more than 10,000 disease conditions is currently available at the NIH Genetic Testing Registry. These tests cover different aspects of risk assessment and provide predictive, diagnostic and prognostic evaluations. 9 Most of these genetic tests are not currently regulated 10 and as a result we still haven’t reached the stage where genetic testing is used as a first line of intervention for suspected genetic conditions. It has been proposed that the widespread adoption of genetic testing will not only lead to a speedy diagnosis making early intervention possible, but will also ease the gruelling experience of what many families call ‘the diagnostic odyssey’.
Lately, there has been a growing trend towards personal genetic testing, including direct-to-consumer genetic testing where consumers can purchase genetic tests directly from a provider without involving healthcare professionals. However, due to concern over their accuracy and effectiveness, these are currently of limited use. Even so, more than 12 million people have had their DNA analysed with these tests, largely to satisfy their curiosity than for their healthcare needs. 11 The availability of these genetic testing services has rendered an individual’s personal genetic information accessible, and needs to be accompanied by parallel social, legal and economic accommodations. One such adaptation is the Genetic Information Nondiscrimination Act (GINA) in the US, which makes it illegal for most health insurers and employers to use an individual’s genetic testing results. In the EU, the newly implemented General Data Protection Regulation (GDPR) that became effective this year, also covers similar privacy issues regarding access to genetic information. Many countries are still trying to figure out how to balance the two conflicting issues surrounding genetic information. On one hand, the more information that we gather, the faster our research developments will take place translating into innovations that will benefit the population at large. After all, the strength of genomics lies in analysing massive amounts of data that it would need access to. On the other hand, there is a valid and growing concern over the protection of an individual’s data, especially when it involves its possible exploitation by large multinational healthcare and information-based companies.
A fundamental view in genomics argues that our capacity to sequence the entire genome in advance of a disease manifestation will have the most profound implications for healthcare, allowing us to not only predict susceptibility and predisposition, but perhaps allowing us to prevent the affliction completely. This is one of the reasons why genetic testing is currently the most developed use of genomics in clinics. We have been able to use small-scale genetic technologies that target specific genes for a long time now, as in the case of newborn screening that started four decades ago in the US with phenylketonuria. Genomic technologies are now allowing us to expand the breadth and depth of our probing.
“Jack: Yes, but you said yourself that a severe chill was not hereditary.
Algernon: It usen’t to be, I know – but I daresay it is now. Science is always making wonderful improvements in things.” ― Oscar Wilde, The Importance of Being Earnest
This article was specialist edited by Sonya Frazier and copy edited by Madeline Pritchard.
References
- ‘Genetics The Future of Medicine’; NIH https://www.genome.gov/pages/educationkit/images/nhgri.pdf
- ‘What is Genomic Medicine?’, NIH https://www.genome.gov/27552451/what-is-genomic-medicine/#al-2
- ‘How can I access Preimplantation Genetic Diagnosis?’; Genetic Alliance UK http://www.geneticalliance.org.uk/information/services-and-testing/how-can-i-access-preimplantation-genetic-diagnosis/
- ‘The “New Genetics” in Clinical Practice: A Brief Primer’; J Am Board Fam Med. https://www.ncbi.nlm.nih.gov/pubmed/28484071
- ‘What disorders are newborns screened for in the United States?’; NIH https://www.nichd.nih.gov/health/topics/newborn/conditioninfo/disorders
- ‘Newborn blood spot test’; NHS https://www.nhs.uk/conditions/pregnancy-and-baby/newborn-blood-spot-test/
- http://legacy.screening.nhs.uk/policydb_download.php?doc=470
- ‘What is Genomic Medicine?’, NIH
https://www.genome.gov/27552451/what-is-genomic-medicine/#al-2 - DOI: 10.1038/gim.2017.211
- ‘Regulation of Genetic Tests’; NIH
https://www.genome.gov/10002335/regulation-of-genetic-tests/ - ‘2017 was the year consumer DNA testing blew up’; MIT Technology Review
https://www.technologyreview.com/s/610233/2017-was-the-year-consumer-dna-testing-blew-up/










Thanks for sharing this site and this information and amazing site and information….