Can Antibiotics Actually Make an Infection Worse?
For a long time, the general consensus has been that all bacteria are bad, and the last century or so has seen a great amount of effort put into trying to eradicate them. More recently, however, it has come to light that they aren’t all bad – in fact, most of them are the opposite. Our bodies are home to trillions of microbial cells as well as our own. As it turns out, the oft-quoted ‘fact’ that they outnumber our cells 10:1 is likely to be exaggerated; recent research has suggested that the ratio is closer to 1.3:1, and the balance might even tip in your favour when you go to the toilet 1. These microbes are important in maintaining our health and immune function, and it would seem that indiscriminate killing with broad spectrum antibiotics designed to clear infection could actually make things worse.
Our microbes provide us with vitamins, protect us against diseases and may even keep us happy – and that’s just a handful of the benefits2. Conversely, the microbiota plays an important role in disease too. It has been known for some time that certain members of our microbiota act as a barrier to intestinal pathogens, and antibiotic treatment can impair this barrier function. Clostridium difficile, or C. diff for short, can take advantage of this and cause severe infection as a result3. Besides this, microbes also play a direct role in immunity. In each of us there are proteins expressed by the immune system called pattern recognition receptors, or PRRs. PRRs recognise molecules present on the surface of invading pathogens resulting in a chain of events which stimulates an immune response. Our microbiota interact with PRRs too, acting as a kind of ‘training’ for our immune system, priming it to respond faster and more robustly4.
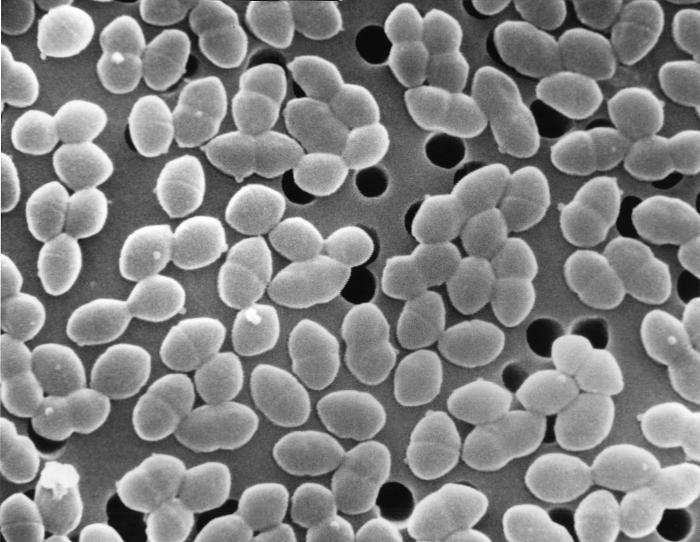
SEM of E. faecalis – a common gut commensal which can also cause severe disease Credit: Janice Haney Carr CDC
These microbes protect us in other ways, too. A team at the Salk institute in La Jolla, California recently discovered a strain of E. coli which protects against muscle wasting caused by infection. In infections like sepsis in humans, muscle wasting can result in serious complications or even kill. The researchers identified a group of mice who appeared to be resistant to muscle wasting during infection and speculated that their microbiome might be actively protecting them. Sure enough, they identified a strain of E. coli present in the resistant group that wasn’t present in the normal mice. Furthermore, when the normal mice were given this E. coli strain orally and infected with intestinal pathogens, they maintained their muscle and fat mass5.
Antibiotic treatment may, therefore, reduce the numbers of these protective bugs and exacerbate infection as a consequence. This is only scratching the surface – in the last couple of years the number of scientific papers investigating the role of the microbiota in immunity has risen dramatically, and some scientists are beginning to think of bacteria as an essential part of our immune system. In some ways it makes sense that our microbes protect us, after all, we provide them with a nice warm, nutrient dense place to live.
Hailed as wonder drugs, antibiotics are well known for their ability to treat disease. But considering their effect on our bacteria and the protective functions they provide, can they actually make us more susceptible to certain infectious diseases6?

Amoxicillin capsules – commonly prescribed first line antibiotic Credit: Maksym Kozlenko Wikimedia Commons
Recent research would seem to suggest that this is at least true in mice. A 2014 study demonstrated that treating mice with a combination of antibiotics and then infecting them with the flu virus resulted in an impaired immune response. Treated mice stayed ill for longer and had lower levels of protective antibodies in their blood7. Moreover, a more recent study demonstrated increased susceptibility to herpes infection in treated mice8. This impairment of immune responses by antibiotic treatment has also been demonstrated with bacterial infections. Depletion of gut microbiota in mice using the same method as the flu study resulted in a stunted response to Staphyloccocus aureus and Streptococcus pneumoniae infection. This study found a component of the cell wall, called peptidoglycan, from commensal bacteria in serum, and that decreased levels of this after antibiotic treatment correlated with impaired immune function. It appears that this effect is due to the absence of priming or ‘training’ of PRRs by the microbiota following antibiotic use, but other factors are also likely to play a role9. Essentially, the antibiotic-treated immune system is more like a couch potato than a highly tuned athlete.
Whether or not similar results can be demonstrated in humans remains to be seen, but given the convincing results in mice and the crucial role of bacteria in immunity, it might just be possible that antibiotics can indeed make us more susceptible to infection. What does this mean for antibiotic use? This is concerning, and should at least provide a boost to public health initiatives encouraging more responsible antibiotic use, especially in countries like the US where antibiotics are available over the counter. This research can be useful for health practitioners too, perhaps those tasked with handing out prescriptions should be more cautious, especially for minor infections.
Antibiotics are a necessity and have been a permanent fixture on the WHO’s list of essential medicines for years, and for good reason. Considering the ongoing crisis of resistance, more effort and investment needs to be put into development of new drugs. But the impact they can have on our health and immunity must also be considered; alternatives that target only the pathogen and leave our microbiota intact are needed. Hopefully, our microbes’ role in immunity will be fully unravelled in the coming years, providing the catalyst for a move towards more specific antimicrobials.
This article was specialist edited by Kathryn McGinnis and copy edited by Nina Divorty
References
- https://www.sciencenews.org/article/body%E2%80%99s-bacteria-don%E2%80%99t-outnumber-human-cells-so-much-after-all
- http://www.nytimes.com/2015/06/28/magazine/can-the-bacteria-in-your-gut-explain-your-mood.html
- https://www.newscientist.com/article/dn23061-faecal-bacteria-cocktail-treats-superbug-infection
- http://www.the-scientist.com/?articles.view/articleNo/43379/title/The-Sum-of-Our-Parts/
- :http://www.salk.edu/news-release/superhero-microbiome-bacteria-protect-against-deadly-symptoms-during-infection/
- Blaser M.J. (2014). Missing Microbes. Harper Collins. New York
- Influenza A original paper: Ichinohe, T., Pang, I. K., Kumamoto, Y., Peaper, D. R., Ho, J. H., Murray, T. S., & Iwasaki, A. (2011). Microbiota regulates immune defense against respiratory tract influenza A virus infection. Proceedings of the National Academy of Sciences of the United States of America, 108(13), 5354–5359.
- http://medicalxpress.com/news/2016-02-antibiotics-susceptibility-sexually-transmitted-infections.htm
- Original paper on S. aureus/S. pneumoniae: Clarke, T. B., Davis, K. M., Lysenko, E. S., Zhou, A. Y., Yu, Y., and Weiser, J. N. (2010) Recognition of peptidoglycan from the microbiota by Nod1 enhances systemic innate immunity. Nat. Med. 16, 228–31










If you want to take a great deal from this post then you have
to apply these techniques to your won web site.