Perovskites – the best material you’ve never heard of

Perovskites are one of the most common materials that exist on Earth, in fact the lower mantle (a layer between the earth’s surface and core) about 660 km below your feet is composed of 93% perovskite1. They have a wealth of electrical, magnetic and optical properties, which have led to many everyday uses.
Applications range from helping the British Navy defeat the Nazis in WW2 as sonar (sound, navigation and ranging) devices to motion sensors in stores, and thermal imaging devices used by firefighters2 3. Still, their true potential is just being realised in the fight against global climate change.
What makes perovskites so useful?
Perovskites are a large family of materials that all have the same general formula, ABX3, and similar structures. The A and B are positively charged metal atoms while the X is a negatively charged oxygen atom (red in Figure 1). Of the metals, A is larger and sits in the centre, with B metal atoms forming a cube around it, which are surrounded by connecting oxygen atoms on every side, and mid-way through the cube of B atoms, which gives the general perovskite structure.
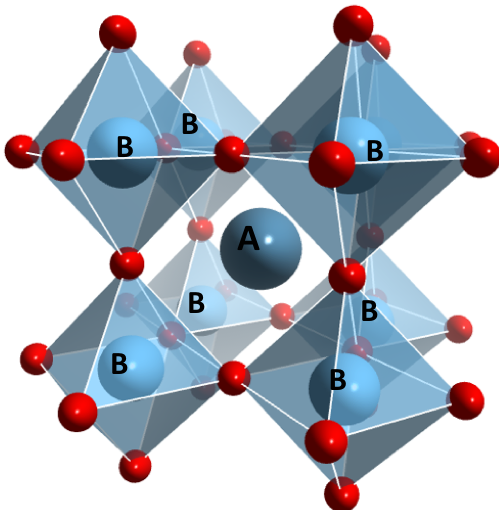
The B atoms form an eight-sided structure, known as an octahedron, with oxygen atoms at its corners. This repeats infinitely in all directions to give a massive three-dimensional lattice structure, like a potato waffle or pie crust.
These octahedrons can tilt and shift, altering the properties of the material. This is a well-known property in materials science, where small changes to the atomic structure can be amplified as dramatic changes in the properties of the bulk solid. Further changes to the solid properties and behaviour can be brought about by choosing different metals4 5.
Changes in properties arise because the atoms moving around in the solid are charged. Movement of charged atoms changes the distribution of electrical charges in the solid. This forms what are known as electrically polarised solids (imagine a solid block with positive and negative charges on opposite ends). If you were to apply an electric field across the solid, the charges align with the field, similar to magnets. Interestingly, once you remove the electric field, the polarisation remains. This is called a ferroelectric solid and finds application as random-access memory (RAM), an essential component in computers6.
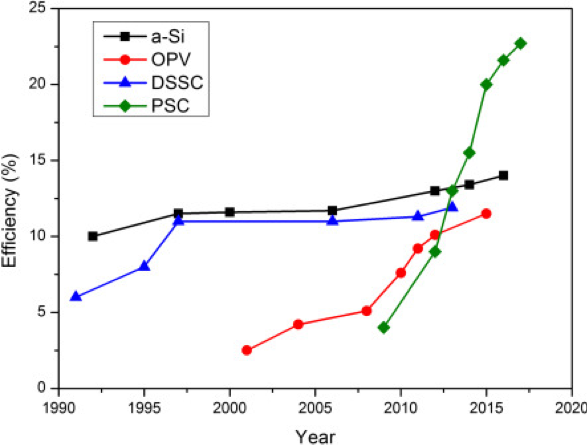
Recent discoveries have shown that some perovskites can also exhibit this electrical polarisation upon exposure to light. This has led to them being used as the light-harvesting layer of perovskite solar cells (PSC). PSCs are being developed for energy conversion devices. These perovskite solar cells absorb almost all visible wavelengths of light and exhibit exceptional power conversion efficiency. Compared to other conventional cell devices (amorphous silicon (a-Si), organic photovoltaics (OPV), dye-sensitised solar cells (DSSC)), PSCs show the greatest improvement in the last 5 years7.
The large-scale production of perovskite solar cells would provide a cheap, versatile source of energy of clean, renewable energy. Although not a sole candidate for future energy production, perovskites will play a significant role in the fight against climate change. From history to modern technologies, perovskites are still making their mark on our society.
Edited by Frankie Macpherson
References
- Dr Mike Glazer in the 2017 Bragg Lecture: www.youtube.com/watch?v=v9bMEUr2II4
- J.Mater.Chem.C,2018,6,10121—10137 (motion and thermal sensing devices
- https://www.chemistryworld.com/features/perovskites-beyond-solar-cells/3010071.article#/(Sonar devices)
- Thomas H. Courtney, 1997. “Fundamental Structure-Property Relationships in Engineering Materials“, Materials Selection and Design, George E. Dieter
- Dr Mike Glazer in the 2017 Bragg Lecture: www.youtube.com/watch?v=v9bMEUr2II4
- Eshita, T., Tamura, T., and Arimota, Y., Ferroelectric Random Access Memory (FRAM) devices
- www.osapublishing.org/prj/fulltext.cfm?uri=prj-2-5-111&id=298642










