Embryo Gene Editing: Changing Life As We Know It
In November 2018 at an international scientific meeting, Chinese scientist He Jiankui claimed he created the world’s first genetically edited babies, a set of twins named Lulu and Nana. Emphasis lies on the word ‘claimed’. A number of establishments, including the hospital that allegedly approved his project and the university where he is an associate professor, have denied any involvement. Chinese authorities have since launched an investigation. Though his claims (which he also shared on YouTube1) remain to be verified, Jianku received stark criticism from around the globe.
Manipulating embryonic DNA is highly controversial since it can radically alter human evolution and society as we know it. Nonetheless, with the aid of recent and rapid scientific and technological advances, scientists have been pushing the legal and ethical boundaries of this competitive research landscape. With the field entering unexplored territory, it is important to understand not only the science behind modifying human embryos, but also its risks, alongside the legal, ethical, and social issues it raises.
What is Germline Gene Therapy?
Deoxyribonucleic acid, or DNA, is the hereditary material found in humans and almost all organisms. Unique sequences of DNA make up genes, some of which act as instructions for cells to make molecules called proteins. Genes, which dictate our unique characteristics, are required for us to develop and live. In turn, genetic changes, which may be inherited or acquired over one’s life, can directly cause disease or increase one’s risk of developing diseases. Gene therapy, which involves delivering genetic material into patients’ cells, can be used as a form of treatment in diseases with a genetic component. There are two types of gene therapy: somatic and germline.
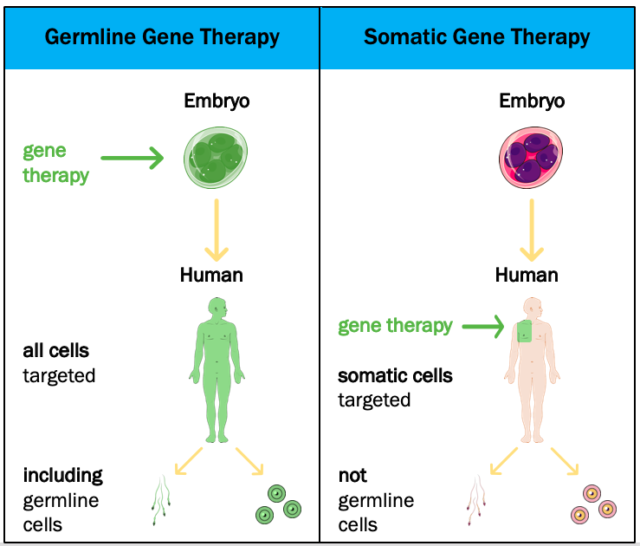
Somatic gene therapy is the introduction of genes into the non-reproductive cells in our bodies, called ‘somatic cells’. While germline gene therapy is the introduction of genes into our reproductive cells – sperm in men and eggs in women. Reproductive cells carry an individual’s genetic information and pass it onto their offspring. Hence, modifying the genetics of germline cells causes any changes that are made to be passed down to future generations. In contrast, somatic gene therapy in an individual will lead to genetic alterations in all their cells, except for reproductive cells. Changes are, therefore, uninheritable. Whether or not genetic modifications can be inherited is critical in understanding the scientific potential and risks of performing gene therapy in an embryo.
What is Embryo Gene Therapy?
Embryo gene therapy is a type of germline gene therapy. Modifying genes in a developing embryo will affect virtually all of the embryo’s cells, including those that will eventually become reproductive cells. Hence, any genetic changes in an embryo will one day be inherited by their offspring. This means diseases with a genetic basis could be eradicated within families and in theory, over time, throughout the entire human population. This heart-warming utopia, however, is perhaps solely that – a utopia: a perfect, disease-free society restricted to our imaginations. In reality, the safety and ethical concerns of embryo gene therapy set a more harrowing scene.
What are the Safety and Efficacy Concerns?
First and foremost, gene therapy carries serious safety risks, particularly in terms of its adverse or ‘off-target’ effects. Concerns related to the off-target effects of traditional gene therapies range from inducing toxicity to eliciting potentially fatal immune responses, to activating cancer-causing genes. All of these unwanted and dangerous effects have previously occurred in human gene therapy trials2. It should be noted though that developments in the field have led to safe and effective therapies, and, as of 2012, there were 9 gene therapies approved worldwide, with this number increasing.
Another notable and recent development is the gene-editing tool, CRISPR/Cas9, the tool used by Jianku to edit Lulu and Nana’s genes. Sometimes described as ‘molecular scissors’, CRISPR/Cas9 is one of the most precise and perhaps safest gene therapy techniques to date; and in late 2018, the first-in-human trials received approval by the FDA and EU. Though trials are currently underway, some researchers are calling for caution as CRISPR’s off-target effects are still being investigated and remain to be fully elucidated3.
With respect to embryo gene therapy, gene-editing tools have been utilised in studies in pigs, sheep, cattle and non-human primates4, and the majority of studies have reported little to no off-target effects5. The arguably favourable safety profile of the technique in animals has driven several research groups to conduct embryo gene therapy in human embryos – in contrast to Jianku’s experiment, these embryos were either non-viable and/or discarded after the experiment; they were not implanted into women. The first study detected a number of off-target events6, while in the three studies that followed789 no off-target effects were detected. However, identifying off-target effects relies on software predictions and statistical assumptions – one study stated, ‘the embryos likely carried off-target mutations undetected by our genotyping, and the off-target effects thus merit further inquiry.’10. The results from Jianku’s study have not yet been published. But according to a CRISPR geneticist, Gaetan Burgio, who attended the scientific meeting, it remains uncertain whether or not off-target effects have occurred11. Although off-target effects may not be toxic or translate to life-threatening situations, the potential is still there.
In addition to off-target events, unintended knock-on effects induced by modifying a gene could lead to physiological benefits or enhancements but likewise to unexpected side effects. Such changes are impossible to predict, even through extensive animal research. Though moving research into humans inevitably carries risks, it should be remembered that the risks of embryo gene therapy extend beyond just one individual, and to potentially thousands.
In terms of its efficacy, it is important to consider the potential of embryo gene therapy to generate ‘genetic mosaicism’. Genetic mosaicism is when within one individual certain cells have a different genetic makeup to other cells. This phenomenon can occur as the result of embryo gene therapy, whereby a portion of the embryo’s cells are successfully edited but others carry unedited versions of the gene, rendering the therapy ineffective. Mosaicism has been a prevalent issue across animal and human studies. Furthermore, Jianku reported unedited versions of the targeted gene have been detected in Lulu.
Jianku intended to confer Lulu and Nana HIV resistance by editing a gene known as CCR5. This in itself raises serious ethical questions. Rather than conducting a potentially life-saving gene therapy, a gene was edited to protect them against a disease which, nowadays, has a number of highly successful treatment options available. Furthermore, Lulu still remains susceptible to HIV due to genetic mosaicism. Thus, gene therapy put her health at risk without a guaranteed benefit. As full details of the investigation emerge, it appears Jianku lied on consent forms and forged ethics review papers. Jianku clearly disregarded the ethical implications of his actions, raising questions over the legality of his experiment.
Legality of Embryo Gene Therapy
With respect to legislation, a worldwide consensus regarding the legal status of embryos remains unclear. In Europe, a human being is granted human rights ‘once born’. Thus, an embryo is not endowed with human rights. This seemingly clashes with ‘Everyone’s right to life shall be protected by law’ (European Convention of Human Rights). However, since an embryo is not yet born, it is not recognised as a human being, and the law does not apply. Although embryos are not legally defined as human beings, their potential to become one is recognised to varying degrees. In 26 countries, abortion is still illegal. In countries that permit in-vitro fertilization (IVF), there are cases of legal action against fertility clinics where couples have sued for wrongful death after their unused embryos were destroyed. In addition, some countries have banned embryonic genetic testing, citing ethical grounds.
The potential to become a human being is perhaps the most prevalent legal issue in embryo gene therapy. While genetically screening IVF embryos means a portion are discarded and will never become humans, embryo gene therapy means altering the genetic makeup of embryos that will become humans. This is particularly important to consider, as effects of embryonic gene editing may not appear until childhood or even much later in life, and this individual wouldn’t have been given the option to consent or refuse therapy. Furthermore, deleterious effects could be inherited and carried over for generations.
Accordingly, embryonic research across the globe is largely banned or restricted, and germline gene modification is currently prohibited through legislation or guidelines in 29 countries. This includes the majority of EU countries, Canada, Australia, and across Asia (China included), and its implementation is restricted in the USA. Referring to Jianku’s research, the Vice-Minister for Science and Technology in China stated, ‘The genetically edited infant incident reported by media blatantly violated China’s relevant laws and regulations. It has also violated the ethical bottom line that the academic community adheres to. It is shocking and unacceptable.’12.
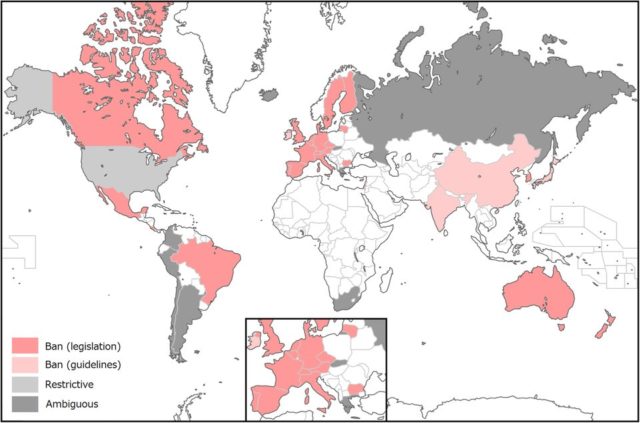
However, recent statements from scientific bodies suggest they appear to be changing their stance. The US National Academies of Science, Engineering and Medicine13 and the UK’s Nuffield Council on Bioethics14, have released statements calling for the need to establish new governance over human genome editing, stating germline gene editing ‘could be morally possible’. Additionally, Japan has introduced draft guidelines to permit the use of gene-editing tools in human embryos for research purposes15. Recently a research group in the UK has received approval to conduct human embryo gene editing with the prerequisite that embryos are discarded after 14 days16. Despite the extensive global backlash Jianku received, this apparent shift highlights a change in attitudes. Surprisingly, public opinion (depending on the poll and country) also seems to show some signs of approval17. Given these trends, it is important to carefully consider and understand the legal and ethical implications of embryo gene therapy.
Concluding Thoughts
Embryo gene therapy places human evolution into the hands of the global scientific community. It probes our legal and ethical boundaries, and shifts the question from ‘if it could have been a human’ to ‘when it will become a human’. While animal studies can offer insight into the effectiveness and safety of embryo gene therapy, the effects in human embryos will only become evident once performed and become apparent in people who do not yet exist. With research carried out irrespective of their will, the ramifications of such actions paint a dystopian future for humankind. Though there are certainly infinite reasons to resist the practice of genetically manipulating embryos, it appears that even in the near future, this practice may become a part of our society. Should embryo gene therapy reveal itself to be a safe and effective treatment, it holds the potential to cure diseases, save countless lives, and extend life-spans. However, the implementation of gene therapy treatments raises concerns outwith the realms of medical practice. Would this therapy be accessible to all? Or benefit only those with the financial means to access it? What would these changes mean in terms of how our society is structured? With the field swimming in a wave of impelling momentum, perhaps most pertinent is for these questions to be addressed before it is too late – if it is not so already.
This article was specialist edited by Niamh Armstrong and copy-edited by Emily May Armstrong.
References
- https://www.youtube.com/watch?v=th0vnOmFltc
- https://whyy.org/segments/the-trial-that-changed-clinical-trials-human-gene-therapy-and-the-death-of-jesse-gelsinger/
- https://www.wired.com/story/a-flawed-study-shows-how-little-we-understand-crisprs-effects/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4737318/#path4648-bib-0098
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4493275/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4417674/
- https://www.nature.com/articles/nature23305
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4870449/
- https://www.cell.com/molecular-therapy-family/molecular-therapy/fulltext/S1525-0016(18)30378-2
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4870449/
- https://twitter.com/GaetanBurgio/status/1067664916545335296
- https://www.theguardian.com/science/2018/nov/29/work-on-gene-edited-babies-blatant-violation-of-the-law-says-china
- http://www.nationalacademies.org/gene-editing/consensus-study/index.htm
- http://nuffieldbioethics.org/news/2018/heritable-genome-editing-action-needed-secure-responsible
- https://www.nature.com/articles/d41586-018-06847-7
- https://www.crick.ac.uk/research/labs/kathy-niakan/human-embryo-genome-editing-licence
- https://www.geneticsandsociety.org/internal-content/cgs-summary-public-opinion-polls#igmdata











1 Response
[…] Photo source: https://the-gist.org/2019/05/embryo-gene-editing-changing-life-as-we-know-it/ […]