DIY Gene Therapy: A New Trend in Biohacking

Biohacking has been a longstanding theme throughout science fiction, from Mary Shelley’s classic novel Frankenstein to Rupert Sanders’ recent action-filled movie Ghost in the Shell. But biohacking, or ‘do-it-yourself (DIY) science,’ is no longer an abstract or futuristic concept. In fact, it is becoming a growing trend, garnering increasing attention.
Encapsulated in its name, biohacking is broadly defined as using science and/or technology to hack living organisms. It encompasses two main spheres: hacking non-human organisms and hacking humans. Unconventional and innovative projects include genetically modifying plants to glow in the dark and using pigment-producing bacteria to develop biodegradable ink. With regards to biohacking oneself, this can range from altering one’s diet to making direct changes to our genome. Recently, several individuals have shared videos on social media showing themselves self-administering gene therapies, thereby propelling biohacking into a whole new arena – DIY gene therapy. While enthusiasts cite their right to self-experimentation, health care professionals, academics, and government agencies have been outspoken critics and are working to highlight the potential dangers. If DIY gene therapy continues to grow as a movement, learning about the field, exploring different perspectives, and discussing its implications are crucial to promote understanding of a sci-fi concept that has now become reality.
Gene Therapy: An Overview
Gene therapy is a technique that involves introducing genetic material into cells, aiming to compensate for missing or defective genes as a treatment for disease. While this may sound relatively straightforward – swap a disease-causing gene with a new healthy one – in practice, it is remarkably complex.
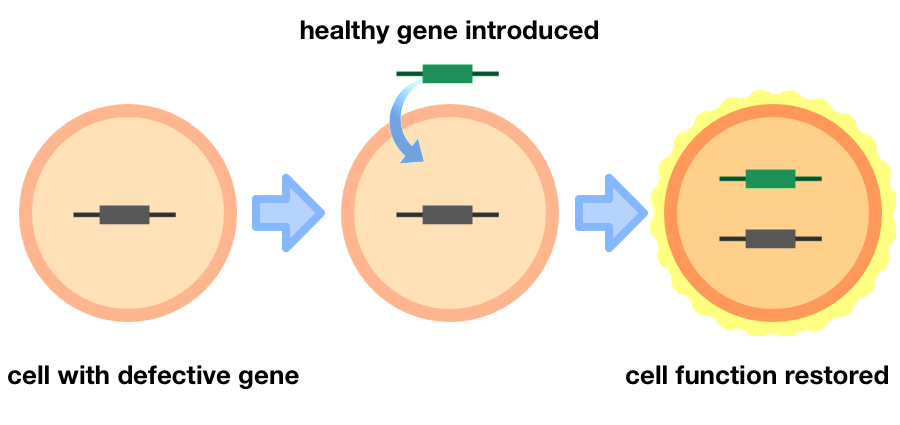
A basic guide to gene therapy: the introduction of genetic material into cells can compensate for abnormal genes to restore cell function as a treatment for disease. Credit: Adapted from immortal coin images.
The first issue, and a fundamental requirement of gene therapy, is that genetic material must enter a cell’s nucleus for it to work. Therefore, it must be able to withstand and penetrate the body’s physical barriers, such as enzymes in the blood that readily degrade genetic material and the cell wall that surrounds every cell in our body. Hence, gene-carrying vehicles (also known as ‘vectors’) are required to facilitate the transfer of genetic material into cells.
Vectors fall into two main categories: non-viral and viral delivery systems. Viruses are an ideal delivery vehicle for genes since these microscopic agents possess unique mechanisms to infect or ‘transduce’ cells. Viral-based vectors, derived from viruses, such as the adenovirus, retrovirus, and herpes simplex virus (HSV), are genetically modified to reduce their toxicity while retaining their ability to get inside cells. As a result, viral gene therapies benefit from relatively high gene transduction efficiency compared to non-viral gene therapies.
Non-viral vectors include chemically synthesised agents that form a complex with DNA and plasmids. Plasmids are circular, double-stranded DNA molecules that can automatically replicate. They occur naturally in bacteria, from which they can be isolated and manufactured to carry a gene. Although non-viral vectors carry fewer safety risks than viral vectors, off-target effects and toxicity are a ubiquitous concern in gene therapy. This highlights a further issue, namely that genetic material is ideally only targeted to cells relevant to the disease.
Finally, once the gene is inside the nucleus of its target cells, a gene must be functioning well enough and for long enough to produce the desired clinical outcome. Failure to address or poor understanding of any of the aforementioned requirements can lead to ineffective treatments. More importantly, it can also lead to dangerous and even fatal outcomes, both of which have occurred in clinical trials. Overall, these factors highlight the challenges of developing a successful gene therapy as well as the gravity of clinical testing.
A History of Gene Therapy: Early Clinical Trials and Current Status
In 1990, 4-year-old Ashanthi DeSilva became the first person to take part in a gene therapy trial 1. She was born with a rare genetic disorder called adenosine deaminase severe combined immunodeficiency (ADA SCID), which arises from inheriting two faulty copies of the gene that encodes the enzyme ADA. With all other treatment options exhausted, she entered the trial and received a genetically modified retroviral vector carrying a functioning version of the gene. Today, Ashanti still requires low dose enzyme replacement therapy, but, as the first of its kind, the trial was arguably a success. However, not all gene therapy trials have shared this outcome. In 1999, Jesse Gelsinger, who suffered from a rare metabolic disorder, ornithine transcarbamylase (OTC) deficiency, was the first person to die in a gene therapy clinical trial 2. Within hours of receiving an adenovirus vector carrying the OTC gene, Jesse developed an immune reaction and passed away four days later. In a 2002 trial, four out of nine infants treated with a retrovirus-mediated gene therapy for SCID developed leukaemia, and the trial was halted after two years 3. With gene therapy trials facing backlash and rising safety concerns, it is of little surprise that the first gene therapy, Glybera, was not approved until 2012.
Since the approval of Glybera, the number of gene therapy clinical trials has steadily been increasing, with six gene therapies now approved in the western world. As scientists have gained a deeper understanding of how vectors interact with the human body and taken a more apprehensive approach to pre-clinical and clinical testing, significant progress has been made in gene therapy. The field has expanded from rare single-gene (also known as ‘monogenic’ disorders) to the treatment of complex disorders, which arise from an array of genetic and environmental factors. In line with this, gene therapy trials are being conducted in a variety of complex disorders, including cancer, cardiovascular, infectious, and neurological diseases. In fact, two of three approved gene therapies in the USA are indicated for different types of blood and skin cancer. With a total of 2,597 on-going gene therapy clinical trials in 2017, the number of approved therapies is expected to rise 4. It is important to note, however, that the majority of trials remain in the early phases of clinical development, with only an estimated 4% at later stages. This highlights the rigorous testing that gene therapies undergo during development. However, this carefully regulated process has now been challenged with the recent rise of DIY gene therapy experiments that have been circulating on social media.
DIY Gene Therapy: Recent Cases and Perspectives
In October 2017, Josiah Zayner became an online sensation after injecting the gene-editing tool CRISPR into his forearm at a synthetic biology conference in San Francisco. In his experiment, Zayner used CRISPR to cut the gene myostatin, with the aim of promoting muscle growth in his body. In a recent interview, the self-proclaimed social activist defended his live-streamed event stating, ‘One of my big problems with academic and medical science is you read lots of these papers. Lots of stuff, we cured X or we did X, but it won’t be available to the general public for 10, 20, 30, 40 years. To me, that seems ridiculous. How do you expect this technology go forward if they aren’t testing, playing around it?’ 5 The intention of his self-experimentation was not to share a major scientific breakthrough or demonstrate the potential therapeutic value of gene therapy. Rather, Zayner hoped that knocking down his own gene in a public forum would break down barriers surrounding scientific research. The aim was to show the public that they can take science into their own hands and that gene editing tools should be available to them, not regulated by research institutions or pharmaceutical corporations. He believes individuals should be entitled to edit their own genes, so it is perhaps unsurprising that he is CEO of The ODIN, a company that sells DIY gene therapy products and kits online to the public.
The freedom to explore biology and the right to self-experimentation are concepts rooted in the ideology of biohacking. Through scientific investigation, enthusiasts seek to gain a deeper understanding of human biology and, in turn, hack the system with self-improvement in sight. Self-experimentation is not explicitly illegal and, in fact, has a history in the field of medical research. Thus far, eight scientists have been awarded Nobel Prizes for their work related to self-experimentation. While such experiments may harness the potential to advance research and prompt scientific breakthroughs, Zayner’s stunt and the cases that followed highlight how DIY gene therapy and its public accessibility challenge these perspectives.
A few weeks after Zayner posted his video, Tristan Roberts (a 28-year-old computer programmer) live-streamed himself injecting a plasmid carrying an antibody into his stomach, a product prepared by the company Ascendance Biomedical 6. Roberts expressed hope of potentially curing HIV, which he had been diagnosed with 6 years earlier. In February 2018, the CEO of Ascendance, 28-year-old Aaron Traywick, injected himself with an experimental herpes treatment containing a modified version of HSV at a biohacking conference in Austin, Texas. Roberts and Traywick, who unlike Zayner have little or no medical experience, hoped their experiments could drive research forward or even cure their diseases, potentially saving patients around the world. Though luckily neither were seriously harmed immediately after, their experiments did not produce the results they had wished for. But, could their results have driven research forward and truly had the impact they believed? And, to what extent were these experiments socially responsible? This is a particularly important question given that Traywick passed away 3 months later, in May 2018. Although, equally important to note is that his cause of death remains publicly unknown.
DIY Gene Therapy: Dangers and Concerns
In terms of scientific value, it is difficult to draw conclusions from data of a single person, arguably undermining any results, with self-experimentation also prone to bias. Furthermore, any treatment requires approval before it can be prescribed, meaning adherence to the drug development process and its corresponding feasibility and safety studies are necessary for a treatment to reach patients. In addition, live-streaming such experiments on social media and the resulting disproportionate hype can hinder public understanding. This puts the delicate relationship between regular citizens and scientists into jeopardy, a relationship which the research enterprise, through public engagement, has paradoxically been seeking to build. Finally, providing inaccurate information and false hopes can obscure the dangers involved in DIY gene therapy, potentially putting peoples’ safety at risk.
After watching Traywicks self-administered herpes treatment, Zayner echoed this sentiment in a Facebook post stating, ‘Looking at my actions in the past, which unfortunately did include a public injection in a semi-ridiculous manner, I want to apologize, in that I could have inspired people to think I was doing things on a whim when I was not… All of this is not to say I am against self-experimentation or treatment… What I am against are biohackers and sketchy companies misleading people into believing they have created cures for diseases or that cures could be created so easily.’ 7 Furthermore, shortly after his self-experimentation, Roberts announced, ‘…after having my optimism thoroughly crushed and trampled, it’s with an unburdened heart that I am announcing that I am dissociating myself from Ascendance Biomedical at least until the CEO is removed. Aaron Traywick is, by most definitions, a scam artist.’ 8
As evidenced by the fatal outcomes of past clinical trials, self-experimenting with untested gene therapies should not be taken lightly, an important message at a time when such therapies are accessible to the public. Though DIY gene therapy products and kits supplied by The ODIN require certain expertise as well as access to facilities that the majority of people do not have, they are still readily available online. Moreover, Ascendendance Biomedical offers gene therapy research compounds to anyone who signs up online, with no restrictions in place. With items labelled ‘not for human consumption’ and ‘non-pathogenic,’ regulatory agencies have been put in a difficult situation, and it remains a grey area whether self-experimenting with such unapproved treatments is punishable by law. Nonetheless, the FDA issued a warning that the sale of DIY gene therapy products and kits is against the law, citing concern over the safety risks involved 9. Previously, the German government has also issued a statement that ordering these kits and conducting experiments out with a licensed genetic engineering facility can lead to a €50,000 fine or up to three years imprisonment 10. However, it remains to be seen whether and what legal actions will be taken, with Ascendance Biomedical stating April 2018 as their earliest release for products.
Concluding Remarks
While the recent social media stunts bring the potential dangers of DIY gene therapy to a forefront, it is important to remember that these dangers are not necessarily shared throughout the vast field of biohacking. Biohacking does not equate to taking life-threatening risks, with the mission of ‘establishing a vibrant, productive and safe community’ lying at its core 11. This is reflected in the recent development of a draft code of ethics, devised to act as a framework to help guide DIY biologists and future research 12. However, DIY gene therapy highlights the new ethical and legal questions that biohacking may raise, as the movement searching to democratize research is only just beginning to grow.
This article was specialist edited by Emily May Armstrong and copy-edited by Kirsten Woollcott.
References
- https://www.biomol.com/the-first-human-gene-therapy.html?id=1457
- https://www.nytimes.com/1999/11/28/magazine/the-biotech-death-of-jesse-gelsinger.html
- https://www.sciencedaily.com/releases/2008/08/080807175438.htm
- http://www.abedia.com/wiley/continents.php
- https://www.theatlantic.com/science/archive/2018/02/biohacking-stunts-crispr/553511/
- http://www.bbc.co.uk/news/world-us-canada-41990981
- https://www.facebook.com/josiah.zayner/posts/10103180183663617
- https://diyhpl.us/wiki/transcripts/2018-02-11-ascendance-biomedical-update/
- https://www.fda.gov/BiologicsBloodVaccines/CellularGeneTherapyProducts/ucm586343.htm
- https://geneticliteracyproject.org/2017/02/14/biohacker-crackdown-germany-threatens-gene-editing-hobbyists-with-fines-jail/
- https://diybio.org/
- https://diybio.org/codes/











Genetic Engineering is a set of technologies used to change the genetic makeup of cells, including the transfer of genes within and across species boundaries to produce improved or novel organisms. It will help to cure many genetic issues in future. Thank you for all these wonderful and informative blog. This will be very helpful for my research studies for Clinical research fellowship. This is very beneficial for me. Thank you once again. Keep sharing such informative blogs.
Despite its futuristic outlook and results biohacking remains a largely unsupervised, dangerous if not illegal movement. Though the research and results produced by biohackers is ground breaking, it also breaks several ethical codes on human experimentation and endangering live of several humans.
If we are doing it to ourselves then no one should be allowed to say anything about it. We understand the risks and most if not all of us make sure we have enough background to at least semi-know what we are doing to ourselves and how to do it unlike some of those attention-seeking idiots on youtube so the regards for ethical codes on human experimentation need to take a hike and shove off. If someone wants to be a part of an experiment then they should be allowed to. Don’t pharmaceutical companies do this on a regular basis along with doctors who use people as guinea pigs and give them 10 different prescriptions over a year or so to see what works and doesn’t work?
Very informative post.
I found this article very helpful.
I found this article very helpful.
Very informative post found here thanks for sharing.