How Competition for Blood Has Shaped the Evolution of Arthropods

Blood-feeding (haematophagy) is an ancient behaviour, having evolved more than 20 different times independently in arthropods. Blood-feeding parasites likely evolved as soon as the avian and mammalian cardiorespiratory system did; approximately 200 million years ago. Arthropods have overcome many evolutionary hurdles to exploit this ‘blood-feeder’ niche.
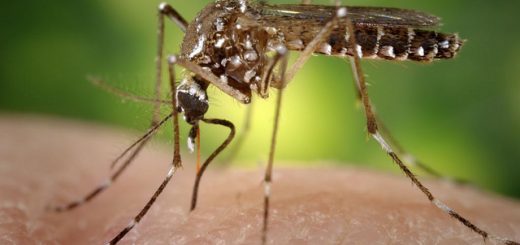
With over a million described species, Arthropoda is the most numerous animal taxon on earth. Included in this diversity are many independent lineages of blood-sucking haematophages, which generally belong to three groups: arachnids, insects, and crustaceans. Blood-feeding has been observed in five orders of insects: Hemiptera, Phthiraptera, Diptera, Siphonaptera, and even Lepidoptera. Some species of arachnid (Acari, the mites and ticks) and copepod (Crustacea) are also haematophages. A plethora of strategies to locate and survive on a host have evolved in Arthropoda. Blood-feeders must not only find a host and suitable location to draw blood, but also possess the correct apparatus to access it, evade defences and ultimately digest their meal. The specificity required for the haematophage niche may be a reason why only 12,000 species of arthropods are blood-feeders 1.
Blood-feeding may have evolved for different reasons depending on the lineage. Consuming blood allows a parasite to increase their fitness by gathering additional amino acids, carbohydrates, and salts. Some lineages, such as the mosquitoes (Culicidae) require blood primarily for reproductive reasons. Only female mosquitoes will consume blood as a source of protein for the yolks of their developing eggs. Blood proteins are essential for vitellogenesis, the large-scale production of yolk-protein precursors (YPPs) in the fat body (the insect version of a liver) of the mother. YPPs are then taken up by the developing eggs. For this reason, haematophagy is crucial to the reproductive cycles of mosquitoes. In Calyptra, a genus of blood-feeding moths, research has shown that individuals do not enhance their longevity from blood-meals. Rather, there is some evidence suggesting that salt is transferred from the male to the female during copulation, either to enhance egg production, or to restore salt supplies required for oviposition 2. Blood-feeding is therefore a multi-purpose strategy that extends beyond direct benefits to individual fitness.
The first step in taking a blood-meal is locating a suitable host. In the blood-feeding mosquito, Aedes aegypti, host location relies heavily on olfactory receptors located at the tip of the stylet. In 2015, a Korean research team found that two olfactory receptors were activated in response to volatile compounds present in blood 3. Olfaction (smell) and gustation (tasting) are the primary modes of host location in terrestrial arthropods, e.g. Haller’s organ in ticks is a grouping of 10 olfactory and gustatory receptors that bind vertebrate associated compounds such as carbon dioxide and fatty acids. Carbon dioxide has been found to play a role in triggering questing behavior (positioning in anticipation of host contact) in hard ticks. Other compounds that may be involved include volatile glandular secretions and metabolic residues from the gut. Furthermore, some evidence suggests that ticks differentially infest cattle, possibly as a result of variations in their immune response. Ticks that select stressed, lactating, or sick cows may have a selective advantage due to a weakened host immune response 4. Haematophages therefore detect more information from a host than just their presence. Assessing host fitness may be a highly adaptive trait in blood-feeding arthropods if their feeding-success varies with the condition of their host.
After a host has been successfully located, specialised mouth-parts are required to begin feeding. These parts are often thin, serrated proboscides which are adapted to piercing skin. The most common methods of insertion include cutting, drilling and back and forth movements. Specifically, in insects, ticks, and mites these mouth parts are specialized in simultaneously administering saliva to the wound and sucking up blood by a pumping mechanism located in the head or body. There are many ancestral mouthparts that could give rise to specialized blood-feeding apparatus. Mouthparts of haematophagic arthropods could have evolved from ancestral, non-blood-feeding mouthparts specialized for biting, piercing, and sponging. Skin-piercing mouthparts often are derived from similar fruit-piercing ancestors as is the case for haematophagic Lepidoptera.
Possibly the greatest evolutionary challenge that blood-feeding arthropods have overcome is the inhibition of host inflammatory and immune responses. As a result of convergent evolution among the many blood-feeding lines of arthropods, various families of protein have been modified and recruited into the salivary proteome to serve similar functions. There are many cases of salivary protein gene duplications and modifications that have resulted in altered or novel blood-feeding functions 5. The functions of most known salivary proteins found in blood-feeders are involved in altering host inflammation, vascular action, platelet activation, and the coagulation cascade. For example, apyrases, a class of enzyme found in all animals which hydrolyses ADP and ATP to AMP, has been recruited into the salivary proteome of bed bugs (Cimex lectularius). In bed bug saliva, apyrases have gained the novel adaptive function of inhibiting coagulation by eliminating blood ADP, which is required for platelet aggregation 6. Furthermore, proteins found in the saliva of haematophagic ticks, Diptera, and triatomine bugs have been shown to bind homeostatic and inflammatory agonists 7. The evasion of host responses through adaptations of salivary proteins in blood-feeding arthropods has been integral to their evolution.
Iron is highly abundant in blood. It is found primarily in the haem groups of haemoglobin, however is an active centre to an array of enzymes. Free iron however has been found to generate reactive oxygen species, which can damage proteins, lipids, and DNA. Haematophagic arthropods have evolved certain mechanisms to increase their survivability when exposed to large quantities iron. The salmon louse for example, expresses increased control of iron transport and storage by iron regulatory proteins, which regulate cellular iron concentrations, specifically in their eggs and reproductive organs. The IRP1 protein has been identified in several invertebrate and mammal species as control of cellular iron concentrations is not limited to blood-feeding parasites. This kind of mechanism however, is a necessary adaptation for haematophagic arthropods, which are exposed to large amounts of iron from blood.
There are several hypotheses that describe how a non-haematophagic arthropod could evolve into an obligate blood-feeder. A non-blood-feeding ancestor could have shifted to a facultative blood-feeding strategy, perhaps by occasionally drawing blood while feeding on skin, or by taking advantage of pre-existing wound sites. There would be a positive selection on phenotypes that best accessed the blood and inhibited host defences. As has been observed in the aforementioned Calyptra moths, wound-site feeding does occur. This suggests that an opportunistic wound-blood-feeding strategy is a plausible ancestral behaviour in some haematophages. Furthermore, this suggests that nectarophages (nectar-eating) organisms with sucking mouthparts are capable of evolving haematophagic strategies. Predators, saprotrophs, seed/fruit piercers, and ectoparasites with biting mouthparts are other possible haematophage ancestors. In contrast to haematophagic butterflies, there is evidence that fleas (Siphonaptera) could have suddenly switched to a blood-feeding strategy. Due to their close relatedness to the moss-eating scorpion flies (Mecoptera), which possess chewing mouthparts, some researchers have suggested that the ancestor of fleas must have switched to a blood-feeding strategy as a result of being introduced to bird and mammal nests in the nest building process 8. Since blood-feeding has evolved independently several times, there is no single evolutionary pathway by which such a strategy can arise. Blood-feeding behaviour in Lepidoptera and Hemiptera were found to originate from plant-feeding behaviour without an animal-feeding intermediate 9. The facultative haematophagy of L. salmonis however, supports the hypothesis that blood-feeding may arise from a gradual transition from skin and/or mucous feeding in some lineages.
Although blood-feeding is successful, having evolved independently many times and persisted through in geological time, it only occurs under certain conditions. One species of mosquito (Wyeomyia smithii) was found to be polymorphic, displaying haematophagy in one population and obligate non-blood-feeding in the rest of their range. Studies of the genetic changes in metabolism suggest there is a tradeoff between the high metabolic cost of anticipating a blood meal in W. smithii and the reproductive benefits of consuming blood 10. Additionally, a more opportunistic lifestyle is an advantage of a non-blood-feeding strategy. If the metabolic costs of haematophagy outweigh the reproductive benefits in terms of fecundity, evolution will favor a return to non-blood-feeding, as was observed in W. smithii. Another trade off occurs when a haematophage acts as a vector for pathogens, a strong, direct cost to host fitness. Due to the strong selective pressure for host defenses to improve, vectors become increasingly specialized, again at the price of a generalist lifestyle.
Obligate blood-feeding is a risky strategy that comes at the price of opportunism. This is likely why such a small number of arthropod species have evolved to be obligate haematophages. Furthermore, the metabolic costs of anticipating a blood-meal may play a role in reverting back to non-blood-feeding strategies. There is no single evolutionary route towards blood-feeding behaviour. Sap-piercing organisms may have switched to blood-feeding directly as they already possess piercing mouthparts, while nectar or liquid feeding ones may have initially fed on open wounds. Other organisms, such as the aforementioned salmon louse likely fed on skin and mucous before digging too deep into the host’s skin and consequently adapted to better digest blood.
Several key adaptations have been observed in blood-feeding arthropods: (1) Olfactory and gustatory receptors allow for fast and efficient host location, (2) Specialized mouthparts have evolved to better access host blood, (3) Salivary proteins mitigate host immunity, (4) Iron regulatory proteins avoid iron poisoning. Blood-feeders have shaped the evolutionary landscape around them by putting strong selective pressures on their hosts, both through blood-feeding itself as well as transmitting harmful pathogens. Haematophages are a powerful tool pathogens can exploit to effectively infect their hosts. This can come at a strong cost to host-fitness, thereby strongly influencing their evolution, as well as our own.
This article was specialist edited by Alexandra Brumwell and copy-edited by Laura Kane.
References
- www.ncbi.nlm.nih.gov/pubmed/22317988
- www.ncbi.nlm.nih.gov/pubmed/22796530
- www.ncbi.nlm.nih.gov/pmc/articles/PMC4549640/
- www.ncbi.nlm.nih.gov/pubmed/21445567
- europepmc.org/abstract/med/23791653
- europepmc.org/abstract/med/23791653
- www.ncbi.nlm.nih.gov/pmc/articles/PMC2889010/
- www.ncbi.nlm.nih.gov/pubmed/22317988
- www.ncbi.nlm.nih.gov/pubmed/22796530
- www.ncbi.nlm.nih.gov/pmc/articles/PMC5798368/









ew it looks like me