Ununpentium – The 115th Element
A new element has been added to the periodic table! Although scientists have as yet found no evidence for adamantium, kryptonite or mithril (and goodness knows, they’re trying their best) there is now evidence to support the existence of element number 115 1.
Ununpentium translates as “one one five… ium” and is only a temporary name. Deciding on a permanent name can be a long process, so until it is chosen, elements are named after their number in the periodic table. Scientists often also refer to this as simply “element 115.”
The periodic table has been growing steadily since Mendeleev published the first version in 1869. Mendeleev is often hailed as something of a genius because he insisted on leaving gaps in the table, in order to accommodate those elements that had not yet been discovered, but which he believed must exist. Back then, there were only 56 known elements, the heaviest of which was uranium, and new elements were found at a rate of about one per year. Since then the gaps have been filled, with the newest and heaviest elements being added at the end of the table.
Ununpentium was first spotted in 2004 2 but The International Union of Pure and Applied Chemistry, along with The International Union of Pure and Applied Physics require, at a minimum, one independent confirmation. Scientists at GSI Helmholtz Centre for Heavy Ion Research in Germany can now provide that confirmation, which is why this can only now be treated as a discovery.
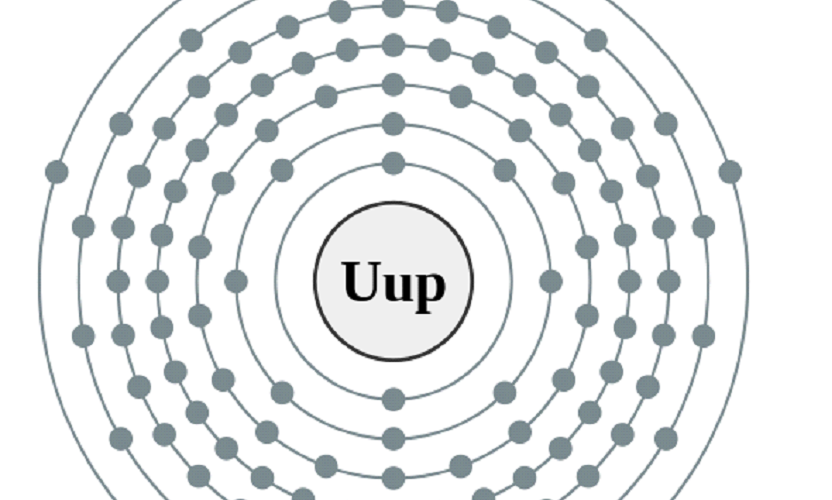
Figure 1. The electron shell diagram for ununpentium is rather pretty, in its way, but would have been a nightmare to sketch out for a high school chemistry exam. (Image credit: Wikipedia; creative commons license.)
It is a homologue of Bismuth – which means it sits in the same column of the periodic table and has similar electrochemical properties – or it would have if not for the fact that it decays so quickly. It has a half-life of just 100 milliseconds, meaning that even if you were to create a metric ton of the stuff, within 2 seconds you would be left with only a gram; the rest would have decayed away into other elements.
Very heavy elements tend to be unstable and extremely short-lived; they break down, or “decay”, into lighter elements very quickly, making it difficult to reliably detect them once they’ve been produced. Their production usually requires heavy ion collisions, in which two heavy atoms are thrown together with immense energy, in the hope that they will fuse into one, even heavier, atom. In this case, the researchers fired calcium atoms (element number 20) at an americium (element number 95) target to create a small number of atoms, each with 115 protons. They then concentrated on measuring the decay products.
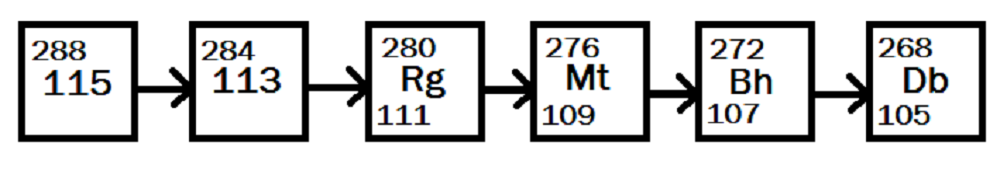
The main decay chain that the researchers considered is shown in figure 2. Each decay from one element to the next involves the ejection of an alpha particle – a helium nucleus (a helium atom without its electrons) – as well as a small burst of light in the form of x-rays. The energies of these x-rays are very well predicted by theory and depend on the elements produced as the initial element decays. By detecting the x-rays, scientists can be more certain of the decay chain they are observing. The element Rutherfordium – named in honour of physicist Ernest Rutherford – (element number 104) was detected using a similar method back in the 1920s.
Figure 2. One possible decay of ununpentium into slightly more normal, considerably more stable elements. Here it decays from Ununpentium, to element 113 (yet to be named), to Roentgenium, to Meitnerium, to Bohrium and finally to the comparably stable Dubnium. All rather exotic, but not so much as Ununpentium.
This, in a sense, is relativity at work. Using Einstein’s famous equation, E=mc^2, the researchers knew that energy (E) and mass (m) are equivalent and that the speed of light (c) is the “exchange rate.” The mass of element 115 is almost, but not quite, the same as the combined mass of element 113 and a helium nucleus. That small difference in mass is converted into energy, in this case in the form of x-rays. So from relativity the researchers knew very precisely what kinds of x-rays to expect, allowing them to watch out for 115’s “energy fingerprint.”
However, 115 isn’t the heaviest confirmed element – this superlative belongs to livermorium (element 116), which was added to the periodic table, along with flerovium (element 114) in 2012 3. Heavy elements aren’t necessarily discovered in order and just because something’s heavier doesn’t always mean it’s harder to produce (or that it decays faster). Other things besides mass will affect stability. Funnily enough, although element 113 occurs in the decay chains for 115, it had not previously been confirmed either – it is likely to be added to the periodic table soon. There is also some evidence for element 118, but it still needs at least one more group of researchers to detect it before its existence can be confirmed.
So, it’s time to replace your periodic table themed mugs and shower curtains and to help come up with new lyrics for Tom Lehrer’s “Element Song.”
References
- D Rudolph et al. Spectroscopy of element 115 decay chains. Physical Review Letters. 2013; 111(11): 112502
- Y Oganessian et al. Experiments on the synthesis of element 115 in the reaction 243Am(48Ca,xn)291-x115. Physical Review C. 2004; 69(2): 021601
- R D Loss and J Cornish. Names and symbols of the elements with atomic numbers 114 and 116 (IUPAC recommendations 2012). Pure Applied Chemistry. 2012; 84(7): 1169-1672