Born Too Early: The Prevention of Preterm Birth

Every year approximately 15 million babies are born too early, of which almost 1 million die from pre-term birth (PTB) complications1. This makes PTB the leading cause of newborn deaths worldwide. In addition to higher mortality rates, babies born prematurely are at an increased risk of long term morbidity, including chronic health problems and neurodevelopmental disorders. The association between the timing of birth and a baby’s health status gained recognition and attention in the 1950s. In 1961, the World Health Organization (WHO) redefined pre-term birth as a baby born before 37 weeks of pregnancy, a definition that holds to this day. While the concept of preventing PTB has existed for more than 50 years, it has been met with limited success and PTB rates continue to rise worldwide2. This remains an important public health issue, alongside the prediction of PTB and neonatal management.
Current pharmacological treatments for preventing an early birth carry major limitations and the use of tocolytics, previously the most common treatment, has steadily declined in recent years3. Tocolytics are labour suppressing agents designed to block the uterine contractions of labour, with evidence showing they are effective in delaying delivery by up to 48 hours4. The use of these drugs would enable medical professionals to transfer patients to special care centres and to administer antenatal corticosteroids to help accelerate foetal development. However, the majority of these drugs remain unlicensed due to controversy surrounding their effectiveness, with no clear evidence showing that they improve foetal or maternal outcomes.5. Furthermore, warnings have been issued regarding the safety of these drugs, as serious adverse effects including death of both mother and baby have been reported6. In line with this, the Royal College of Obstetricians and Gynaecologists guidelines state it is reasonable NOT to use any tocolytic drugs currently available7. In response to the limitations of these agents, research efforts have led to the development of progesterone supplementation, approved by the FDA in 2011. This is currently the only approved drug available for the prevention of PTB in the USA, aimed at women pregnant with a single baby and a history of at least one prior spontaneous PTB8. Though evidence supports its use in this high-risk subgroup, this demonstrates its inaccessibility to other pregnant women. The limitations of current treatments highlight the critical need for novel treatments and strategies for preventing PTB.
A major barrier to the development of novel treatments for PTB as well as predictors, or ‘biomarkers’, is our limited understanding of the mechanisms that control the timing and process of term labour. Both hormonal and inflammatory processes appear to be involved in the stimulation of uterine contractions and concurrent inhibition of uterine relaxation that occur during the normal labouring process9. However, a dominant regulator or mechanism has not yet been identified and for approximately half of PTBs the cause remains unknown.
So why do substantial knowledge gaps in term and preterm labour persist? From a research perspective, a major impediment is the significant interspecies differences in pregnancy. For example, in most sub-primate mammals overt progesterone withdrawal controls the timing of labour, while in humans a sudden drop in progesterone levels is notably absent. Though non-human higher primates are most analogous to humans in terms of pregnancy, ethical and financial considerations limit their widespread use. Furthermore, experts have also suggested the human labouring process and its associated pathologies may be specific to our species10. This presents an even greater challenge to researchers in this field given the major ethical issues surrounding research with pregnant humans.
From a broader societal view, newborn survival and health were not specifically mentioned in the Millennium Development Goals (MDGs)11. The WHO has since released the action plan Every Newborn and the report Born Too Soon, in an attempt to specifically address newborn health concerns and PTB respectively1213. Due to the absence of these topics in the MDGs, they received less attention and lower investment in terms of research, drugs and technologies compared to the issues listed14. However, growing recognition of the substantial burden PTB places on our society has reignited discussion within this field and hopefully will provide answers to the countless questions that remain.
In the last 5 years, PTB has increasingly been classified as a syndrome rather than a disease15. This notion has reshaped the approach to treating and developing novel treatments for prevention of early births. PTB is now considered a final common event that may arise from numerous pathways. This highlights the multitude of underlying causes of PTB such as infection and inflammation, maternal and foetal stress, and cervical and placental disorders. While the hunt for a silver bullet to prevent all PTB will continue, this paradigm shift may prompt new developments targeting specific conditions or subgroups of patients, as seen with the recent approval of progesterone.
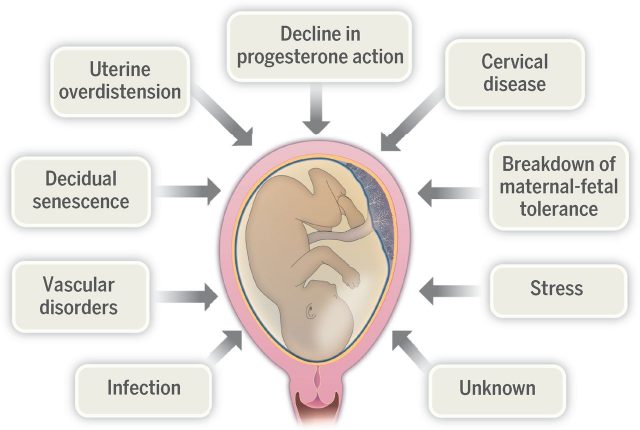
Examples of proposed mechanisms underlying preterm birth, with genetic and environmental factors likely contributing to each mechanism. Credit: Romero, R., Dey, SK. and Fisher, SJ. 2014. Preterm labor: one syndrome, many causes. Science. 2014 Aug 15;345(6198):760-5. via http://science.sciencemag.org/content/345/6198/760/F2
PTB is now also considered to be multifactorial, arising from a combination of genetic and environmental factors. Our understanding of these factors and how they interact has begun to explain and identify risk factors and different observable characteristics or ‘phenotypes’ of PTB. As our understanding of these factors broadens, this provides a springboard for identifying potential biomarkers, pathophysiological processes and novel effective treatments. Furthermore, this addresses the issue of knowing when prevention of PTB is a suitable intervention. It is important to consider that although prevention may be suitable in certain cases, PTB may itself be a desirable outcome in others
The prevention of PTB carries tremendous societal significance given the devastating short and long term socioeconomic costs. The Institute of Medicine in the USA estimates that the annual cost of PTB is $26.6 billion. In the UK, the daily cost of neonatal intensive care is estimated to be £800-1000, not considering the costs associated with long term disability and disease. The emotional impact of losing a baby or the lifelong challenges carers may face bring the social consequences of PTB to the forefront. The power of prevention is further highlighted by improvements in PTB rates that have been achieved by alternatives to pharmacological treatment, such as providing dedicated care and educating women about lifestyle changes that can improve their health and pregnancy. For example, smoking cessation, dietary supplements and monitoring patients at high risk have shown to cause a significant reduction in PTB rates in certain subgroups of women16. While the importance of amenable factors should not be downplayed, the necessity for preventive pharmacological treatments remains equally significant. With the potential to prevent the death and disability of millions of newborns, the development of a wonder drug to prevent PTB remains yet to be achieved.
This article was specialist edited by John Holden and copy edited by Katrina Wesencraft.
References
- http://www.who.int/pmnch/media/news/2012/201204_borntoosoon-report.pdf
- http://www.who.int/pmnch/media/news/2012/201204_borntoosoon-report.pdf
- https://www.rcog.org.uk/globalassets/documents/guidelines/gtg_1b.pdf
- https://www.ncbi.nlm.nih.gov/pubmed/23048010/
- https://www.ncbi.nlm.nih.gov/pubmed/23048010/
- https://www.fda.gov/Drugs/DrugSafety/ucm243539.htm
- https://www.rcog.org.uk/globalassets/documents/guidelines/gtg_1b.pdf
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3218546/
- https://blogs.biomedcentral.com/on-medicine/2016/06/24/examining-the-contribution-of-hormones-and-inflammation-to-preterm-birth/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4493222/
- http://www.who.int/maternal_child_adolescent/topics/newborn/enap_consultation/en/
- http://www.who.int/pmnch/media/news/2012/201204_borntoosoon-report.pdf
- http://www.who.int/maternal_child_adolescent/topics/newborn/enap_consultation/en/
- http://www.who.int/maternal_child_adolescent/topics/newborn/enap_consultation/en/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4191866/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4237124/